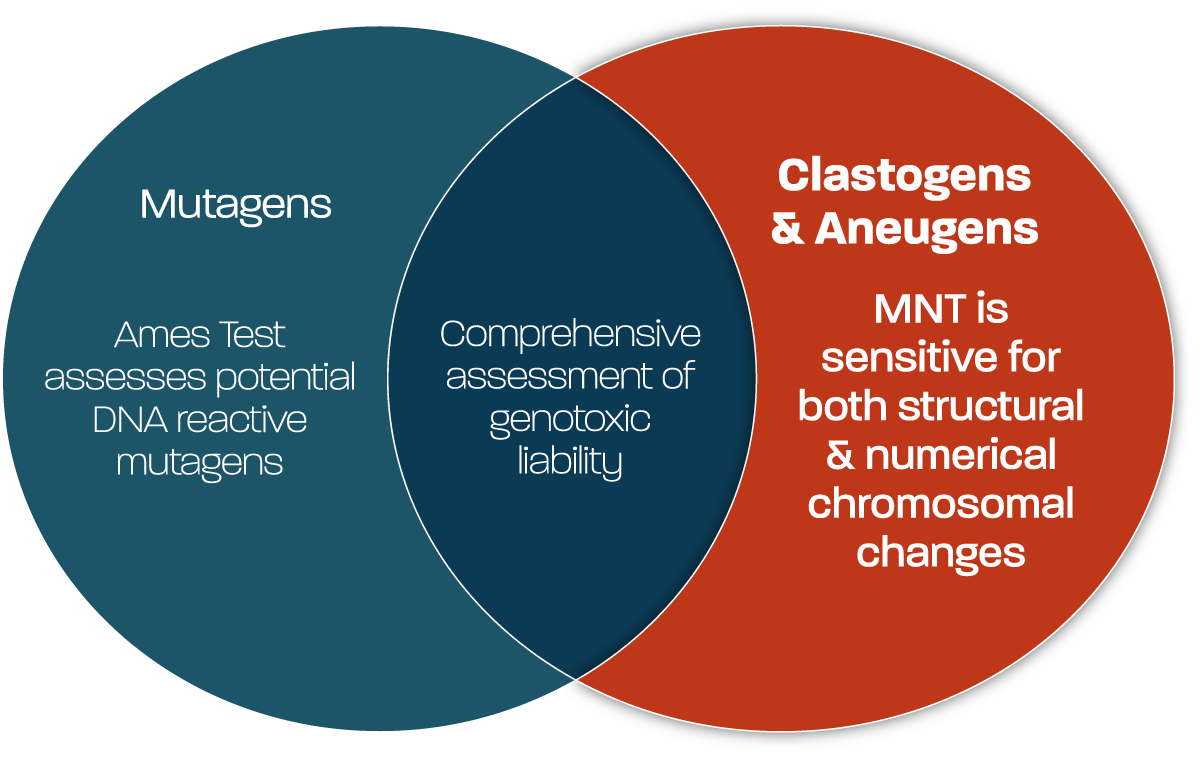
Clastogens & Aneugens
Gentronix offers a range of screening options for the assessment of a test substance, to determine its potential to induce structural (clastogens) and numerical (aneugens) chromosomal damage.
SERVICE INFORMATION
Such studies are an important component in determining overall genotoxic liability of test substances, given both routes of chromosomal damage can occur when a substance is either DNA reactive (potentially also an Ames test positive) or not directly DNA reactive (potentially Ames test negative). The tools available for screening at Gentronix allow detection of both clastogens and aneugens, using study designs that are high-throughput, quick turnaround, using small amounts of test item and capable of differentiating the toxicological mechanism.
Chromosomal damage is most commonly determined through the assessment of whether a test substance causes the formation of micronuclei, or through detection of biomarkers in the nucleus which are precursors to their formation. The majority of global industries now require assessment of test substances for the ability to induce such chromosomal damage using the OECD 487 in vitro micronucleus test. Increases in micronuclei in the cytoplasm of interphase cells are indicative of either chromosome breaks (clastogens), or abnormal segregation of chromosomes during mitosis (aneugens). As such the in vitro micronucleus test can detect both the clastogenic and aneugenic modes of action for a test substance in a single endpoint. Inclusion of pan-centromeric FISH probes, allow further analysis to assess whether micronuclei are centric (resulting from Aneugens) or acentric (resulting from Clastogens).
Whilst the OECD 487 study is most often conducted using fluorescence microscopy to detect micronucleated cells, this requires a higher test substance amount that is typically available at a screening project stage. Gentronix has adapted this method to enable comparable study designs to be performed in a 96-well microplate, analysing cells for micronuclei in an identical manner to an OECD 487 study design. This approach is also compatible with FISH, giving a regulatory assessment for as little as 20 mg, and capable of screening 100s of test substances across responsive timelines.
Alternative approaches, focussed on higher-throughput and fast-turnaround techniques such as flow cytometry MNT can also be applied to assess micronucleus formation at Gentronix, predicting OECD 487 outcome with low compound usage. This platform can also be applied to MutliFlow®️, which focus on the assessment of cellular biomarkers indicting DNA strand breakage, genotoxic stress &/or arrest of cells in mitosis and induction of polyploidy. Together, these markers are used in a statistical toolbox to assess whether test substances are likely to present a clastogenic, aneugenic or no genotoxic liability in a screening discovery stage.
These screening options available at Gentronix, provide a range of options that can be tailored to specific project requirements, to give a comprehensive assessment of chromosomal damage potential at the early screening stage. This informs best next steps, reducing genotoxic liabilities and promoting project success. Talk to our toxicology team for help with design the best study package for you needs.