All Mechanisms
Gentronix offers a range of screening options for the assessment genotoxic liability in early discovery, focussing on the hazard within a chemical series using minimal test substance quantities in rapid, high-throughput screens.
SERVICE INFORMATION
Whilst the regulatory genetic toxicology battery focus is assessment of safety of test substances by assessing through mechanistic endpoints to detect mutagens, clastogens and aneugens; early-stage discovery can often benefit from approaches designed to first and foremost screen for genotoxic hazard, with mechanistic determination an additional benefit. Substances resulting in gene mutations, structural or numerical chromosomal changes, also trigger DNA damage response & repair pathways, making these useful biomarkers for assessing potential genotoxic hazard in a high-throughput, fast and lower cost manner.
Gentronix developed DNA damage response screening assays using the GADD45a gene, coupled with reporter proteins of either GFP (GreenScreen HC) or luciferase (BlueScreen HC). These assays, using the TK6 cell line, enable high-throughput assessment of genotoxicity, with >90% concordance with in vivo genotoxicity test outcomes, >90% sensitivity for genotoxic carcinogens and >90% specificity. This highly accurate screening assay enables genotoxic liability to be screened out early in a discovery, without the risk of high false positive rates.
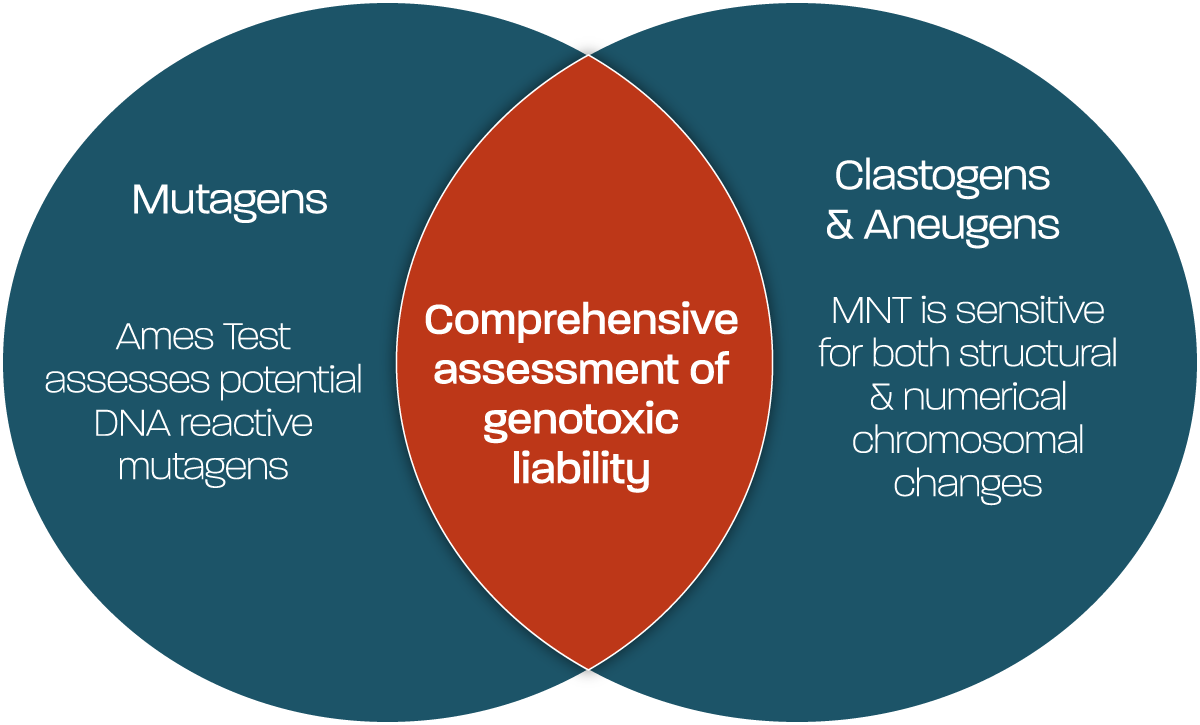
Using multiple biomarkers within a single flow cytometry-based experiment, the MultiFlow®️️ assay can be used to detect genotoxic stress, as well as distinguish between clastogenic and aneugenic modes of action. This assay has considerable utility as a follow-up to positive in vitro results, but its high-throughput compatibility and low compound requirement mean it is also ideally suited as a primary screening tool; capable of generating fast predictions of genotoxic mechanism and mode of action.